In Focal Segmental Glomerulosclerosis (FSGS)
Elevated Proteinuria Increases Risk of Disease Progression1
Endothelin-1 and angiotensin II are key mechanisms of disease progression in FSGS2:
- Working in tandem via their receptors to amplify podocyte injury
- Causing injury resulting in glomerular filtration breakdown
- Increasing proteinuria
Epidemiology of FSGS
- FSGS is a leading glomerular cause of kidney failure3
- Affects those of all ages and represents a significant proportion of glomerulopathy diagnoses4,a
- Is prevalent in patients with recent African ancestry, with up to 80% of biopsies identifying FSGS as the cause of idiopathic nephrotic syndrome5,b
- In adults, identifying FSGS requires biopsy. Further evaluation may be required to differentiate the classification of primary, genetic, or secondary FSGS, or FSGS of undetermined cause
(FSGS-UC)6
aAnalyses conducted from the NEPTUNE cohort; n=441 proteinuric patients (≥0.5 g/24 hrs) undergoing kidney biopsy; NEPTUNE=Nephrotic Syndrome Study Network.
bBased on US data.
Elevated proteinuria correlates with accelerated decline in kidney function and reduced survival probability in FSGS7-9
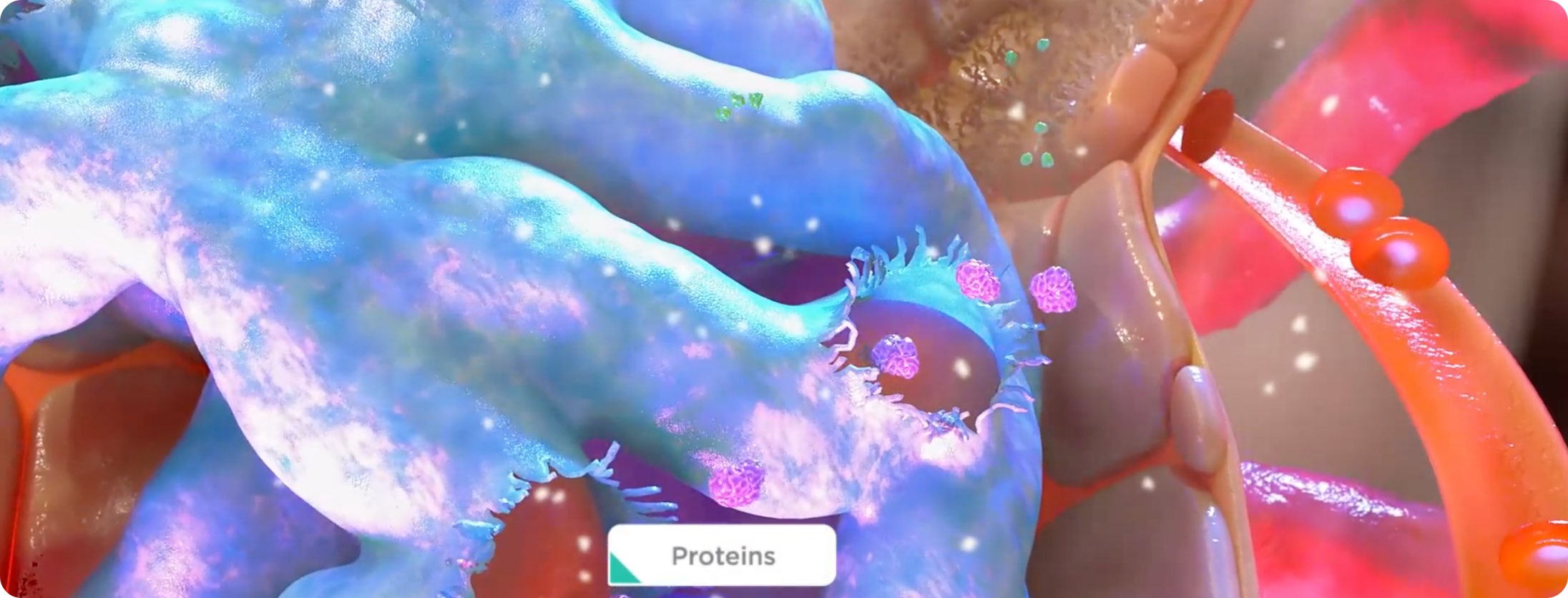
FSGS is characterized by glomerular injury resulting from podocyte damage.10
Reduction of proteinuria to remission and partial remission thresholds can significantly improve
TARGET PROTEINURIA GOAL11,12,c
<1.5 g/g
Achieving this goal correlates with extended kidney survival
cFSGS partial remission threshold defined as <1.5 g/g with a 40% reduction from baseline.
Significantly extended survival in patients with FSGS who achieved <1.5 g/g and 40% decrease in PCR12,d
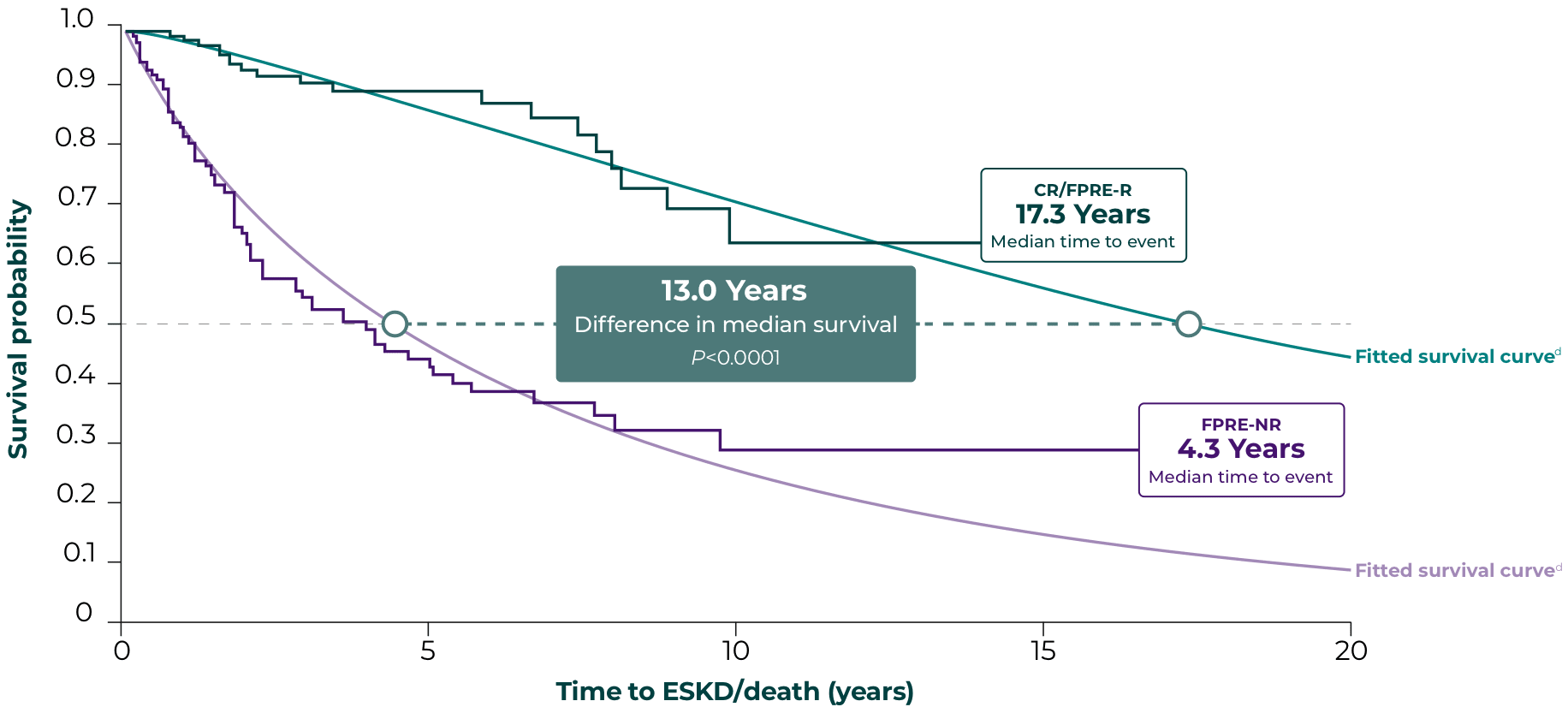
Endpoints based on PCR follow-up 6 to 12 months from first nephrotic-range PCR value:
CR/FPRE-R, PCR <1.5 g/g and 40% decrease in PCR
FPRE-NR, not achieving CR/FPRE
Reducing proteinuria from nephrotic-range baseline to <1.5 g/g correlated with a 13-year extension in median survival probability vs patients who did not reach this thresholde
CR=complete remission; eGFR=estimated glomerular filtration rate; ESKD=end-stage kidney disease; FPRE=FSGS partial remission endpoint; FPRE-NR=FPRE-non-responder; FPRE-R=FPRE-responder; PCR=protein-creatinine ratio.
dTime to ESKD or death was analyzed using accelerated failure time modeling of the Weibull distribution and Kaplan-Meier estimates of cumulative incidence.
eBaseline pertains to first nephrotic-range proteinuria value (≥3.0 g/g) at or after disease onset.
Based on data from the National Registry of Rare Kidney Diseases (RaDaR), a UK Kidney Association initiative, in 270 patients with FSGS (150 adults age 18 and older and 120 pediatrics) with long-term data collection and comprehensive follow-up, representing a nationwide database and involving a large proportion of UK renal centers. ESKD was defined as chronic kidney disease stage 5 (confirmed eGFR <15 mL/min/1.73m2 or CKD stage 5 recorded in RaDaR) or receiving chronic dialysis or kidney transplant. Time to ESKD or death was analyzed using accelerated failure time modeling.
Treatment options are limited
There are no FDA-approved drugs indicated for the treatment of FSGS.13
Treatment strategies are based on limited evidence and include6,13:
- Immunosuppressive treatment
- ACEi/ARB
Research is ongoing to help address the needs in patients with FSGS who are at high risk for rapid progression14
ET-1 and Ang II work in tandem via their receptors to exacerbate kidney injury
ACEi=angiotensin-converting enzyme inhibitor; Ang II=angiotensin II; ARB=angiotensin II receptor blocker; ET-1=endothelin-1; FDA=United States Food and Drug Administration.
References:
1. Kohan DE, Barton M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int. 2014;86(5):896-904. doi:10.1038/ki.2014.143 2. Komers R, Plotkin H. Dual inhibition of renin-angiotensin-aldosterone system and endothelin-1 in treatment of chronic kidney disease. Am J Physiol Regul Integr Comp Physiol. 2016;310(10):R877-R884. 3. Rosenberg AZ, Kopp JB. Focal segmental glomerulosclerosis [published correction appears in Clin J Am Soc Nephrol. 2018 Dec 7;13(12):1889]. Clin J Am Soc Nephrol. 2017;12(3):502-517. doi:10.2215/CJN.05960616 4. Gipson DS, Troost JP, Lafayette RA, et al. Complete remission in the Nephrotic Syndrome Study Network. Clin J Am Soc Nephrol. 2016;11(1):81-89. doi:10.2215/CJN.02560315 5. Korbet SM. Treatment of primary FSGS in adults. J Am Soc Nephrol. 2012;23(11):1769-1776. doi:10.1681/ASN.2012040389 6. Kidney Global Outcomes (KDIGO) Glomerular Diseases Work Group. Kidney Int. 2021;100(4S):S1–S276.7. Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry Group. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16(4):1061-1068. doi:10.1681/ASN.2004070593 8. Le W, Liang S, Hu Y, et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant. 2012;27(4):1479-1485. doi:10.1093/ndt/gfr527 9. Reich HN, Troyanov S, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18(12):3177-3183. doi:10.1681/ASN.2007050526 10. D'Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365(25):2398-2411. doi:10.1056/NEJMra1106556 11. Troost JP, Trachtman H, Nachman PH, et al. An outcomes-based definition of proteinuria remission in focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2018;13(3):414-421. doi:10.2215/CJN.04780517 12. Saleem MA, et al. American Society of Nephrology (ASN) Kidney Week virtual, November 4-7, 2027. PC1529. 13. Raina R, Chauvin A, Chakraborty R, et al. The role of endothelin and endothelin antagonists in chronic kidney disease. Kidney Dis (Basel). 2020;6(1):22-34. 14. Nephcure Kidney International. https://kidneyhealthgateway.com/trials-research/. Accessed March 2024.